Anti-diabetic effect of Jobelyn® Dietary Supplement on alloxan-induced diabetic rats and molecular docking of its bioactives Effet antidiabétique du complément alimentaire Jobelyn<sup>®<sup> sur les rats diabétiques induits par l'alloxane et ancrage moléculaire de ses composés bioactifs
Main Article Content
Abstract
ENGLISH
Background: Jobelyn®, containing Sorghum bicolor extract, is promoted as an anti-inflammatory and antioxidant supplement.
Objectives: This study explored the binding of Jobelyn's bioactive compounds to sulfonylurea receptor 1 and its in vivo anti-diabetic potential in rats.
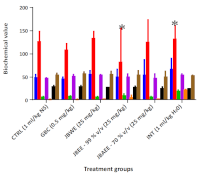
Methods: Molecular docking of the bioactive constituents in Jobelyn® was done to evaluate potential interactions. Thirty alloxan-induced diabetic rats (110-180g) were divided into five groups (n=6) treated with glibenclamide (0.5 mg/mL), Jobelyn® water extract, 99% v/v ethanol extract, or 70% v/v aqueous ethanol extract (25mg/kg), and one diabetic control group took water. A sixth, non-diabetic group received saline. After 21 days, the blood and pancreas were histologically examined.
Results: Quercetin showed the strongest sulfonylurea receptor 1 (SUR1) binding (-9.7 kcal/mol), followed by luteolin and proanthocyanidin, surpassing glibenclamide (-8.1 kcal/mol). All Jobelyn® extracts lowered blood glucose, with 99% ethanol showing the highest (84%) and 70% aqueous ethanol demonstrating the fastest effect. Biochemical and histological profiles also improved.
Conclusion: Jobelyn® showed significant antidiabetic activity at 25 mg/kg, with 70% v/v aqueous ethanol extract showing the fastest response.
FRENCH
Contexte: Jobelyn®, contenant de l'extrait de Sorghum bicolor, est présenté comme un complément alimentaire anti-inflammatoire et antioxydant.
Objectifs: Cette étude a exploré la liaison des composés bioactifs de Jobelyn au récepteur 1 de la sulfonylurée et à son potentiel antidiabétique in vivo chez le rat.
Méthodes: L'ancrage moléculaire des composants bioactifs de Jobelyn® a été réalisé afin d'évaluer les interactions potentielles. Trente rats diabétiques induits par l'alloxane (110-180 g) ont été répartis en cinq groupes (n = 6) traités avec du glibenclamide (0,5 mg/mL), un extrait aqueux de Jobelyn®, un extrait éthanolique à 99% v/v ou un extrait éthanolique aqueux à 70% v/v (25 mg/kg), et un groupe témoin diabétique a reçu de l'eau. Un sixième groupe, non diabétique, a reçu une solution saline. Après 21 jours, le sang et le pancréas ont été examinés histologiquement.
Résultats: La quercétine a montré la plus forte liaison au récepteur de sulfonylurée 1 (SUR1) (-9,7 kcal/mol), suivie de la lutéoline et de la proanthocyanidine, surpassant le glibenclamide (-8,1 kcal/mol). Tous les extraits de Jobelyn® ont abaissé la glycémie, l'éthanol à 99% affichant l'effet le plus élevé (84%) et l'éthanol aqueux à 70% démontrant l'effet le plus rapide. Les profils biochimiques et histologiques se sont également améliorés.
Conclusion: Jobelyn® a montré une activité antidiabétique significative à 25mg/kg, l'extrait d'éthanol aqueux à 70% v/v présentant la réponse la plus rapide.
Downloads
Article Details
Issue
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
Share
References
1. Murray CJL, Aravkin AY, Zheng P, Abbafati C, Abbas KM, Abbasi-Kangevari M, et al. (2020). Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 396(10258):1223-49.
2. Galicia-García U, Benito-Vicente A, Jebari S, LarreaSebal A, Siddiqi H, Uribe KB, Ostolaza H, Martín C. (2020). Pathophysiology of type 2 diabetes mellitus. International Journal Mol Sci. 21(17):6275. doi: 10.3390/ijms21176275
3. Hemat Jouy S, Mohan S, Scichilone G, Mostafa A, Mahmoud AM. (2024). Adipokines in the Crosstalk between Adipose Tissues and other Organs: Implications in Cardiometabolic diseases. Biomedicines 12(9): 2129. Doi: https://doi.org/10.3390/biomedicines12092129
4. Olamoyegun MA, Alare K, Afolabi SA, Aderinto N, Adeyemi T. (2024). A systematic review and metaanalysis of the prevalence and risk factors of type 2 diabetes mellitus in Nigeria. Clin Diabetes Endocrinol. 10(1):43." doi: 10.1186/s40842-024-00209-1.
5. Oh KK, Adnan M, Cho DH. (2020). Network pharmacology of bioactives from Sorghum bicolor with targets related to diabetes mellitus. PLoS One 15(12): e0240873. Doi: 10.1371/journal.pone.0240873.
6. Khalid W, Ali A, Arshad MS, Afzal F, Akram R, Siddeeg A, Kousar S, Rahim MA, Aziz A, Maqbool Z, Saeed A. (2022). Nutrients and bioactive
compounds of Sorghum bicolor L. used to prepare functional foods: a review on the efficacy against different chronic disorders. International Journal Food Prop. 25(1):1045-1062. doi: https://doi.org/10.1080/10942912.2022.2071293.
7. Adebayo AH, Yakubu OF, Egbung GE, Williams OI, Okubena O. (2018). Sub-acute toxicological effects of Jobelyn® on pregnant albino rats. AIP Conference Proceeding. 1954(1):030018. doi: https://doi.org/10.1063/1.5033398.
8. Omorogbe O, Ajayi AM, Ben-Azu B, Oghwere EE, Adebesin A, Aderibigbe AO, Okubena O, Umukoro S. (2018). Jobelyn® attenuates inflammatory responses and neurobehavioural deficits associated with complete Freund-adjuvant-induced arthritis in mice. Biomed Pharmacother. 98: 585-593. doi: 10.1016/j.biopha.2017.12.098.
9. Mursal M, Ahmad M, Hussain S, Khan MF. (2024). Navigating the computational seas: A Comprehensive Overview of Molecular Docking
Software in Drug Discovery. In: Unravelling Molecular Docking-From Theory to Practice. IntechOpen. doi:10.5772/intechopen.1004802.
10. Chavan A, Sabale P. (2020). Molecular docking study and antibacterial activity of novel chalcone derivatives. The Pharma Innov Journal 9(1): 39-42.
11. Chibuye B, Singh SI, Chimuka L, Maseka KK. (2023). A review of modern and conventional extraction techniques and their applications for extracting phytochemicals from plants. Sci Africa. 19: e01585. doi: https://doi.org/10.1016/j.sciaf.2023.e01585.
12. Institute of Laboratory Animal Research (ILAR). Guide for the Care and Use of Laboratory Animals. 8th ed. Washington, DC: National Academies Press; 2011.
13. Locke D. (1983). A new approach to practical acute toxicity testing. Arch Toxicol. 54:275-287. doi:10.1007/BF01234480.
14. Algul S, Ozcelik O. (2025). Comprehensive review of animal models in diabetes research using chemical agents. Lab Anim. 59(3):356-363.
doi:10.1177/00236772241296199.
15. Balamash KS, Alkreathy HM, Al Gahdali EH, Khoja SO, Ahmad A. (2018). Comparative Biochemical and Histopathological Studies on the Efficacy of Metformin and Virgin Olive Oil against Streptozotocin-Induced Diabetes in Sprague?Dawley Rats. Journal Diabetes Res.
4692197. doi:10.1155/2018/4692197.
16. Isa I, Fumiyuki K, Hishashi U. (2023). Comparison of Regulations or Arsenic and Heavy Metals in Herbal Medicines Using Pharmacopoeias of Nine Countries/Regions. Ther. Innov. Regul. Sci. 57(5): 963 - 974. doi: 10.1007/s43441-023-00532-2.
17. Oghenekevwe RM, Olatunbosun SB, Moshood AO, Gloria, AA. (2025). Phytochemical, Heavy Metal Analysis and HPLC Profiling of Jobelyn® - An Herbal Dietary Supplement. Trop Journal Phytochem Pharm Sci. 4(7): 294 - 302. https://doi.org/10.26538/tjpps/v4i7.2
18. Akintemi EO., Govender KK, Singh T. (2024). Molecular Dynamics and Docking Investigation of Flavonol Aglycones against Sulfonylurea Receptor 1 (SUR1) for Anti-diabetic Drug Design. Chem Select. 9(10): (e202302488). doi: https://doi.org/10.1002/slct.202302488.
19. Nair AB, Jacob S. 2016). A simple practice guide for dose conversion between animals and humans. Journal Basic Clin Pharm. 7(2):27-31. doi: 10.4103/0976-0105.177703.
20. Goldner M, Gomon G. (1943). Alloxan-induced diabetes. Endocrinology. 33:297-299.
21. Ighodaro OM, Adeosun AM, Akinloye OA. (2017). Alloxan-induced diabetes, a common model for evaluating the glycemic-control potential of therapeutic compounds and plants extracts in experimental studies. Medicina 53(6):365-74.
22. Rhuada OM, Okoko OD, Ayoola GA. (2019). Antiinflammatory activity of the fruit extracts of Carpolobia lutea G. DON (Polygalaceae). West Africa Journal Pharm. 30(1):54-63. doi:10.60787/wapcp-30-1-169.
23. Famurewa OT, Aje AA, Kolawole BA. (2023). Complementary and alternative medicine use among ambulatory diabetes patients in a SouthWestern tertiary hospital in Nigeria. West Africa Journal Pharm. 31(1):61-71. doi:10.60787/wapcp-31-1-201.
24. Amarakoon D, Lou Z, Lee WJ, Smolensky D, Lee SH. (2021). A Mechanistic Review: Potential Chronic Disease, Preventive Properties of Sorghum. Journal Sc i Food Agri c . 101(7):2641-2649. doi:10.1002/jsfa.10933.
25. Laleye S, Aderiye JBI., David O (2016). Hypolipidemic Activity of Fermented Sorghum bicolor L. Moench) Gruel (Ogi) in Hyperchosterollemiic Rats (Rattus nervegicus). International Journal Biochem Res Rev.9(1):1-15. doi:10.9734/IJBCRR/2016/19659.
26. Owumi SE, Kazeem AI, Wu B, Ishokare LO, Arunsi UO, Oyelere AK. (2022). Apigeninidin-rich Sorghum bicolor (L. Moench) extracts suppress A549 cells proliferation and ameliorate toxicity of aflatoxin B1-mediated liver and kidney derangement in rats. Sci Rep. 12(1):7438. doi: 10.1038/s41598-022-10926-1.
27. Shahidi F, Danielski R. (2024). Review on the Role of Polyphenols in Preventing and Treating Type 2 Diabetes: Evidence from In Vitro and In Vivo Studies. Nutrients. 16(18):3159. doi:10.3390/nu16183159.
28. Martiniakova M, Sarocka A, Penzes N, Biro R, Kovacova V, Mondockova V, Sevcikova A, Ciernikova S, Omelka R. (2025). Protective Role of Dietary Polyphenols in the Management and Treatment of Type 2 Diabetes Mellitus. Nutrients. 17(2):275. doi:10.3390/nu17020275.".